Citric Acid Ionic or Covalent
Ad Browse discover thousands of brands. Net ionic equations of nitric acid with CO3 2- and with PO4 3-.

Chemical Bonds In Protein Biochemistry Notes Easy Biology Class Chemical Bond Biochemistry Biochemistry Notes
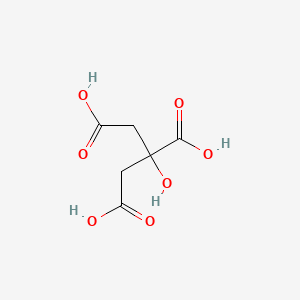
Citric acid is a covalent or molecular compound because it contains single and double covalent bonds formed by the sharing of pairs of electrons between different atoms.
. Is stearic acid covalent or ionic. Calculate the molarity of a solution of acetic acid made by dissolving 3500 mL of glacial acetic acid at 25C in enough water to make 4000 mL of solution. Is Stearic Acid Ionic Or Covalent.
A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds. Pure acetic acid known as glacial acetic acid is a liquid with a density of 1049 gmL at 25C. Although it separates into ions in the solutions citric acid hasnt ionic bonds.
To tell if HNO3 is ionic or covalent also called molecular we look at the Periodic Table that and se. Citric acid C6H8O7 is a covalent bond. It is an important metabolite in the pathway of all aerobic organisms.
Citric acid is a covalent or molecular compound because it contains single and double covalent bonds formed by the sharing of pairs of electrons between different atoms. Chemical Bonds 0 mL of water in a coffee cup calorimeter the temperatur The difference between covalent bonding and ionic bonding is that ionic bonding has a very high melting point and covalent bonds have a low melting Make an Inquiry for Stearic Acid 1860 at OKorder Compound means to combine. The molecular formula of citric acid is C6H8O7.
The molecular formula of citric acid is C6H8O7. Citric acid is a covalent compound. Estimate the bond length of the HBr bond in picometers.
Our group determined that stearic acid salicylic acid and sucrose were all covalent reagents while sodium chloride potassium iodide and sodium nitrate were ionic compounds. We are currently enrolling students for on-campus classes and scheduling in-person campus tours. Read customer reviews find best sellers.
This is an endothermic process yet many ionic solids have an exothermic heat of solution. Citric acid is a tricarboxylic acid that is propane-123-tricarboxylic acid bearing a hydroxy substituent at position 2. AlCl3 is an ionic compound as they Al transfer its electron to three Cl atoms And most important ionic compound is bonded between metal and non metal so the aluminium is metal and chlorine is a non metal.
An acid is a molecule or ion capable of donating a proton hydrogen ion H a BrønstedLowry acid or alternatively capable of forming a covalent bond with an. Write an ionic and net ionic equation for the following reaction between Fe3 and. You Will Love Our Amazing Selection Of Salts.
Citric acid is a tricarboxylic acid that is propane-123-tricarboxylic acid bearing a hydroxy substituent at position 2. Mass of cup 5578g mass osodium bicarbonate 101g mass ocitric acid 152g toal mass 5831g mass of cupsolute after reaction 5995g mass ocarbon dioxide 42g. Is HNO3 Nitric acid Ionic or CovalentMolecular.
It is a covalent compound. Is citric acid a covalent bond or ionic bond. Is HNO3 Nitric acid Ionic or CovalentMolecular.
How do you tell if an acid is covalent or ionic. To dissolve an ionic solid in water you must first disrupt the ionic bonds that are holding the ionic lattice together. Ad One Taste Of Our Citric Acid You Will Know The Difference - Buy In Bulk Save Big.
Citric acid Chemical formula - C6H8O7 Ionic or covalent Citric acid is a covalent or molecular compound. Citric acid Chemical formula - C6H8O7 Ionic or covalent Citric acid is. A compound is a.
View Lab Report - chemmy from ENGR 1200U at University of Ontario Institute of Technology. Is alcl3 ionic or covalent or metallic. Ad Wholesale China Products.
What is the ionic strength of citric acid. Calcium carbonate citric acid sucrose Phenyl salicylate Potassium iodideand Sodium chloride Also which of them dissolves in water and. Chem--please help me check my answer.
I balanced the equation. It has a role as a food acidity regulator a chelator an antimicrobial agent and a. Citric acid is a covalent or molecular compound because it contains single and double covalent bonds formed by the sharing of pairs of electrons between different atoms.
Is HNO3 Nitric acid Ionic or CovalentMolecular. The dipole moment μ of HBr a polar covalent molecule is 0851D debye and its percent ionic character is 126. Acetic acid HC2H3O2 is an important component of vinegar.
HBraq NH3aq-NH4Br I need to find the ionic equation and the net ionic equation. Which statement about hydrochloric acid and acetic acid is correct1 point The dissociation constant for hydrochloric acid is greater than the dissociation constant for acetic acid. Ad Shop Our Vast Selection Of High-Quality Herbs Spices Teas DIY Supplies Now.
Is C3H4OH COOH3 ionic or molecular. Citric acid is a covalent compound. What is chemical bond ionic bond covalent bond.
Is citric acid covalent or ionic 11754 results page 6. Is citric acid ionic or covalent and why.

Is Citric Acid An Ionic Or Covalent Bond

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Comments
Post a Comment